Abstract
Introduction: Ide-cel, a B-cell maturation antigen (BCMA) directed CAR T-cell therapy, has been FDA approved for the treatment of RRMM after 4 prior lines of therapy. While response rates and survival outcomes have been very promising, there is a significant number of patients who do not respond or relapse early after ide-cel. Understanding the characteristics of these patients is important to help guide patient selection and development of novel strategies to improve outcomes. We evaluated factors associated with refractoriness or early progression (≤3 months after CAR T infusion) for patients treated with standard of care ide-cel.
Methods: Eleven U.S. academic centers contributed data to this effort independent of the manufacturer. At the time of data cut off, 240 patients were leukapheresed, 215 patients were infused, 154 patients had at least 3 months follow up available and were the focus of this analysis. Of those, 67 patients had progressed with a progressive event defined as progression or death due to myeloma. We investigated differences in patient, disease, and CAR-T related characteristics by time to progression (≤3 months, >3 months, did not progress) using chi-square or Kruskal-Wallis tests. For factors identified as associated with progression, we performed a multivariable Cox proportional hazard regression analysis to examine the association between these factors and overall survival (OS) and progression-free survival (PFS). Lastly, we summed the number of factors associated with early progression (≤3 months) and examined the association of number of early progression risk factors (0, 1, 2, or 3 factors) with OS and PFS using Kaplan-Meier survival curves and log-rank tests.
Results: Among the 67 patients that progressed after CAR T, median patient age was 61 years (range 36-79 years) and 58% were male. High risk cytogenetics (deletion 17p, t(4;14), t(14;16)) were present in 39% of patients. Patients were heavily pretreated with 6 median (range 4-18) prior lines of therapy and 34% of the patients had received prior BCMA directed therapy. Cytokine release syndrome (CRS) was seen in 74% (grade 3+: 3%) and immune effector cell associated neurotoxicity syndrome (ICANS) in 16% (grade 3+: 4%) of the patients. At data cut off, 48% (32/67) of the patients had progressed ≤3 months (16% (11/67) within 30 days (refractory), 32% (21/67) between 1-3 months), and 52% (35/67) had progressed >3 months post-infusion. With a median follow up of 6.4 months, best overall response rate was 66% with complete remission or better seen in 40% of the patients. Median PFS and OS for the patients who progressed ≤3 months and >3 months were 2.0 and 7.3 months vs 5.3 and 12.5 months, respectively.
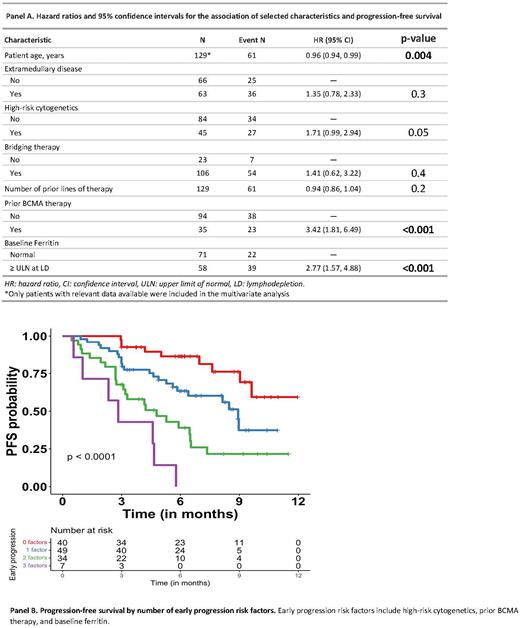
Patients with previous BCMA directed therapy (commercial or on clinical trial), high-risk cytogenetics (particularly t(4;14)), extramedullary disease, and bridging therapy were more likely to have progressed early (≤3 months). Patients with a younger age at infusion, a higher number of prior lines of therapy, and elevated baseline ferritin levels at lymphodepletion (LD) were associated with progression regardless of when patients progressed. Of these variables associated with progression identified in univariate analysis, multivariable analyses showed that patients with younger age (hazard ratio [HR]=0.96 (per one year increase in age), 95% confidence interval [CI]=0.94, 0.99), prior BCMA directed therapy (HR=3.42, 95% CI=1.81, 6.49), elevated ferritin at LD (HR=2.77, 95% CI=1.57, 4.88), and high-risk cytogenetics (HR=1.71, 95% CI=0.99, 2.94) were associated with worse PFS (Panel A). For OS, only patients with prior BCMA directed therapy had significantly inferior OS (HR 4.45, 95% CI=1.72, 11.6). Considering high-risk cytogenetics, prior BCMA directed therapy, and elevated ferritin at LD as factors associated with early progression, patients with two or three of these risk factors had inferior PFS (1 vs 2 vs 3: 8.9 vs 4.8 vs 2.8 months, respectively) and OS (1 vs 2 vs 3: Not reached (NR) vs 12.5 vs 5.9 months, respectively) compared to patients with no early progression risk factors (PFS=NR, OS=11.3 months) (Panel B).
Conclusions: This multicenter retrospective study delineates prior BCMA directed therapy, high-risk cytogenetics, and elevated ferritin at LD as potential predictors of early progression after CAR T-cell therapy for RRMM. Presence of two of three of these factors negatively impact PFS and OS.
Disclosures
Hashmi:JANSSEN: Consultancy; KARYOPHARM: Speakers Bureau; GSK: Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; BMS: Consultancy. Hansen:BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; OncLive: Honoraria; Survivorship: Honoraria. Freeman:Sanofi: Honoraria; Amgen: Honoraria; Janssen: Honoraria, Research Funding; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria. Sidana:Sanofi: Consultancy; Janssen: Consultancy, Research Funding; Allogene: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Magenta Therapeutics: Consultancy, Research Funding; Prothena: Honoraria; Oncopeptides: Consultancy. Sborov:Pfizer: Consultancy; Sanofi: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bioline: Consultancy; Amgen: Consultancy; Abbvie: Consultancy. Wagner:Abbvie Inc.: Other: Partner is currently employed as a Medical Science Liaison . Atrash:Amgen: Research Funding; Celgene: Honoraria, Speakers Bureau; Takeda: Honoraria; Sanofi: Honoraria, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GSK: Honoraria, Research Funding. Simmons:Kite/Gilead: Speakers Bureau. Ferreri:Sanofi: Membership on an entity's Board of Directors or advisory committees; Affimed: Current equity holder in publicly-traded company. McGuirk:Orca Bio: Research Funding; Nextar: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sana: Honoraria; CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial. Locke:ASH: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; CAREducation: Other: Education or editorial activity; Clinical Care Options Oncology: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; Society for Immunotherapy of Cancer: Other: Education or editorial activity; Aptitude Health: Other: Education or editorial activity; Leukemia and Lymphoma Society: Research Funding; ), National Cancer Institute: Research Funding; CERo Therapeutics: Research Funding; Takeda: Consultancy; Sana: Consultancy; Daiichi Sankyo: Consultancy; BMS: Research Funding; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Baz:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; genentech: Membership on an entity's Board of Directors or advisory committees; Shattuck labs: Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria; celgene: Consultancy, Honoraria; karyopharm: Research Funding; Merck: Research Funding. Patel:Janssen, Celgene/BMS, Caribou Sciences, Arcellx, Cellectis, Merck, Pfizer, Karyopharm, Oncopeptides: Consultancy. Alsina:BMS: Research Funding; BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal